Starting IVF can be a very exciting time – it is another step closer to becoming parents. Naturally, you will feel hopeful about a successful outcome but you also need to prepare yourself for around two months of medications, numerous procedures and testing. Please also bear in mind that the success rate of modern fertility treatments is high, but for some of couples, multiple treatment cycles may be necessary. The whole process up to the embryo transfer stage will usually take two to three weeks. IVF & ICSI involve 6 stages:
- Stage 1: Ovarian stimulation and monitoring
- Stage 2: Egg (oocyte) retrieval [Oocyte Pick Up - OPU]
- Stage 3: Fertilisation
- Stage 4: Embryo development
- Stage 5: Embryo transfer
- Stage 6: Luteal phase support
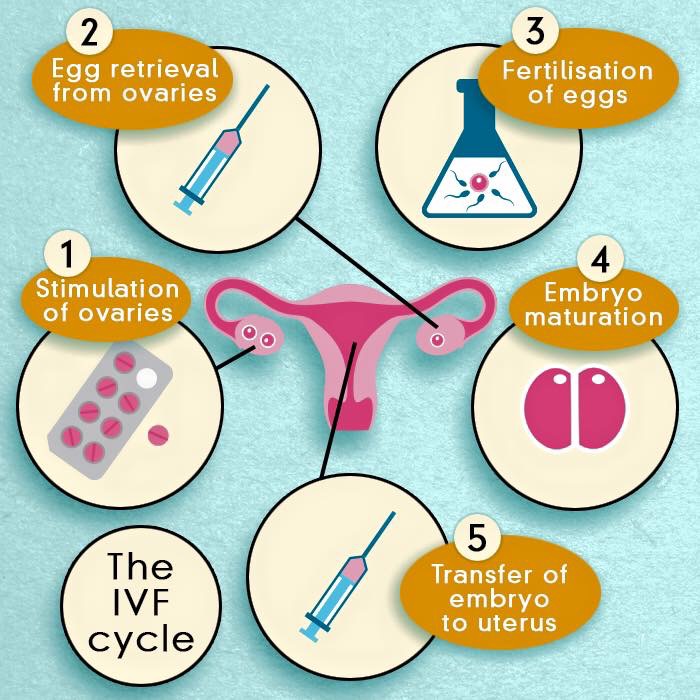
Fig. IVF step by step
Fertility specialist physicians prescribe medications or hormones in order to increase the likelihood of collecting multiple eggs and to control the timing of a patient’s menstrual cycle. The patient and her partner may administer these medications on a daily basis within the privacy of their home. Our staff then monitor the patient’s progress by evaluating the number of ovarian follicles in development through vaginal ultrasound and serial blood samples. A follicle is a sac of fluid in the ovary that may contain an egg (oocyte). Your IVF doctor determines the number and frequency of these tests. Just prior to egg retrieval, a patient takes an additional injectable medication to complete the maturation of the eggs. The retrieval is scheduled only if there are an adequate number of follicles ready.
Oocyte pick Up (OPU) also known as Egg retrieval, is arranged just prior to expected ovulation. OPU is usually performed 36 to 48 hours after the administration of the ovulation inducing drugs hCG or LH.
It is an transvaginal ultrasound-guided procedure in which a long, thin needle is passed through the vaginal wall into the ovary. The physician aspirates the follicles from each ovary and the follicular fluid is collected in test tubes, where the embryologist carefully searches for the eggs. The eggs are cleaned, counted, and placed in an incubator. Later that day, the eggs are fertilized with sperm either by standard insemination (classical IVF) or intra-cytoplasmic sperm injection (ICSI).
OPU is performed under mild sedation, local anaesthesia or, in some cases, general anaesthesia. Injuries during this procedure are extremely rare. Structures near the ovaries, such as the bladder, bowel, or blood vessels, could possibly be injured and require further surgery. Limited bleeding from the ovaries may occur, but the need for transfusion is extremely rare. Infections following transvaginal egg retrieval are also possible, but are rare.
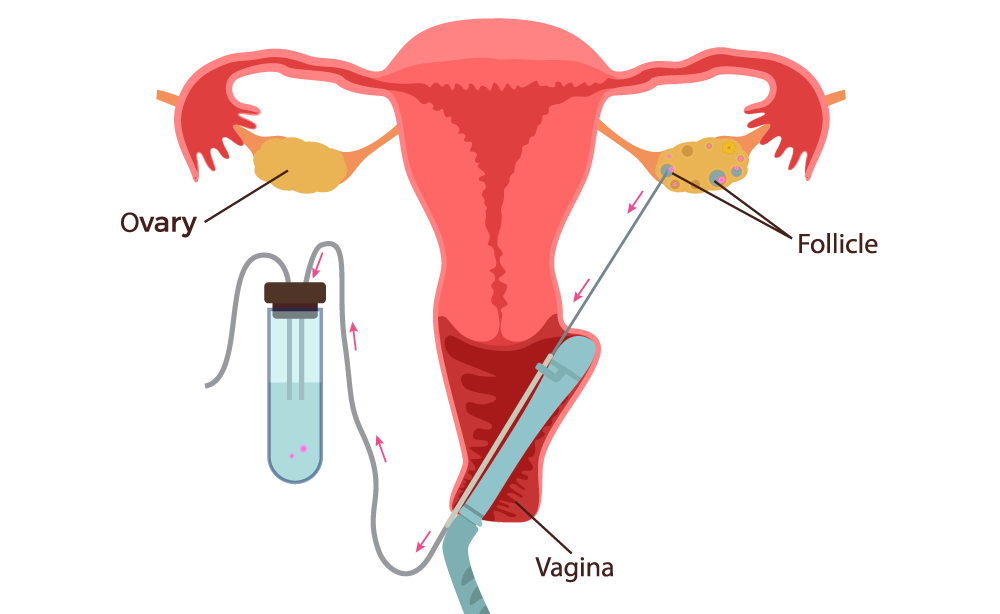
Fig. Schematic presentation of Oocyte Pick Up
Fig. Schematic presentation of Oocyte Pick Up
Not every follicle contains an egg, or some may contain mature eggs which may not be capable of being fertilised. So don’t be surprised if the number of eggs retrieved is less than the number of follicles you’ve been watching develop on ultrasound. The average number of eggs retrieved is between eight and nine and the retrieval process lasts approximately 20–30 minutes.
After the procedure, some women feel a little tender in their abdomen – a hot water bottle may help. You may also feel tired because of the anaesthetic. You will be monitored for a couple of hours before being allowed to go home. You may notice some light vaginal spotting that is red or brown in colour. It is recommended that someone drives you home from the clinic and you may need to take the following day off work because of minor pain or fatigue.
About two hours before egg pick up, a semen sample is collected from the male partner. Two to three days’ abstinence from intercourse/masturbation is preferred prior to the sample collection day. The sperm sample is usually produced by masturbation at the clinic. The sperm is processed to select the strongest, most active sperm. This is called ‘sperm washing’. If sperm are not present in the ejaculate, sperm collection may be attempted surgically.
- If undergoing IVF, the sperm are then placed with the eggs in an incubator set to the same temperature as a woman’s body. The next day, the eggs are examined under a microscope to determine whether fertilisation has occurred and you will be phoned about how many of your eggs have been fertilised. The resulting embryos will be either transferred to the uterus two to five days later, or frozen for later transfer.
- If undergoing ICSI, the eggs are prepared for injection and their maturity confirmed. In what is a delicate laboratory procedure, a single sperm is placed directly into the cytoplasm (the centre) of the egg – hence the name intra-cytoplasmic sperm injection. Fertilisation can then be identified in a similar fashion to IVF after about 20 to 24 hours.
Many fertility patients are curious about what exactly happens in the embryology lab, from the time their eggs are retrieved to the time they are transferred. When people have answers to their questions about the details of embryo development, IVF can become a less mysterious process for them. With that in mind, here is a day-by-day timeline of what happens in the embryology lab in IVF:
- Day 0: This is known as your retrieval day. On day 0, the eggs are retrieved from the follicles that have resulted from your ovarian stimulation. At this time the retrieved eggs are counted and assessed for maturity/quality. The sperm is also prepped and readied for insemination or ICSI of the retrieved eggs. Approximately 4-6 hours after your retrieval, the eggs will be inseminated or injected with sperm (ICSI).
- Day 1: The eggs are evaluated for fertilization, the fusion or combining of the egg and sperm, approximately 16-18 hours after insemination. Normal fertilization is the presence of two pronuclei, one from the egg and one from the sperm. If there are too few or too many pronuclei, that embryo is considered abnormally fertilized. All normally fertilized embryos are put into a special media that mimics the tubal fluid found in the human body.
- Day 2: The embryos are briefly looked at to assess cell division. Most embryos will have between 2-4 cells on day 2. If the embryo has not divided by this time, that embryo is considered non-viable. At this time, embryology will decide if a day 3 or a day 5 transfer will occur. This is typically based on the quality of cell division of the embryos, and the number of embryos available.
- Day 3: Embryos at this stage usually have 6-8 cells. If you are having your embryo transfer, assisted zona hatching, making a breech in the shell of the embryo might be performed. This is also the day embryo biopsy for PGD may occur. If you are not having your transfer, the embryos are placed into new media that mimics the uterine fluid of the human body.
- Day 4: Your embryos continue to grow and develop into a compact ball of cells, about 16 in all, known as a morula. If compaction does not start to occur on this day, blastocyst formation rates are decreased.
- Day 5: On day 5, the embryo develops into a blastocyst. At this stage, it is usually possible to grade the Inner Cell Mass (ICM), the fetal component, and the Trophectoderm cells (TE), the placental component, of the embryo. Embryo transfers are often done on day 5, as well as cryopreservation of any fully expanded blastocysts. Any remaining viable embryos that are not fully developed are cultured on for possible freezing on day 6.
- Day 6: Embryos on day 6 must be either transferred, if transfer was not done on day 5, or frozen.
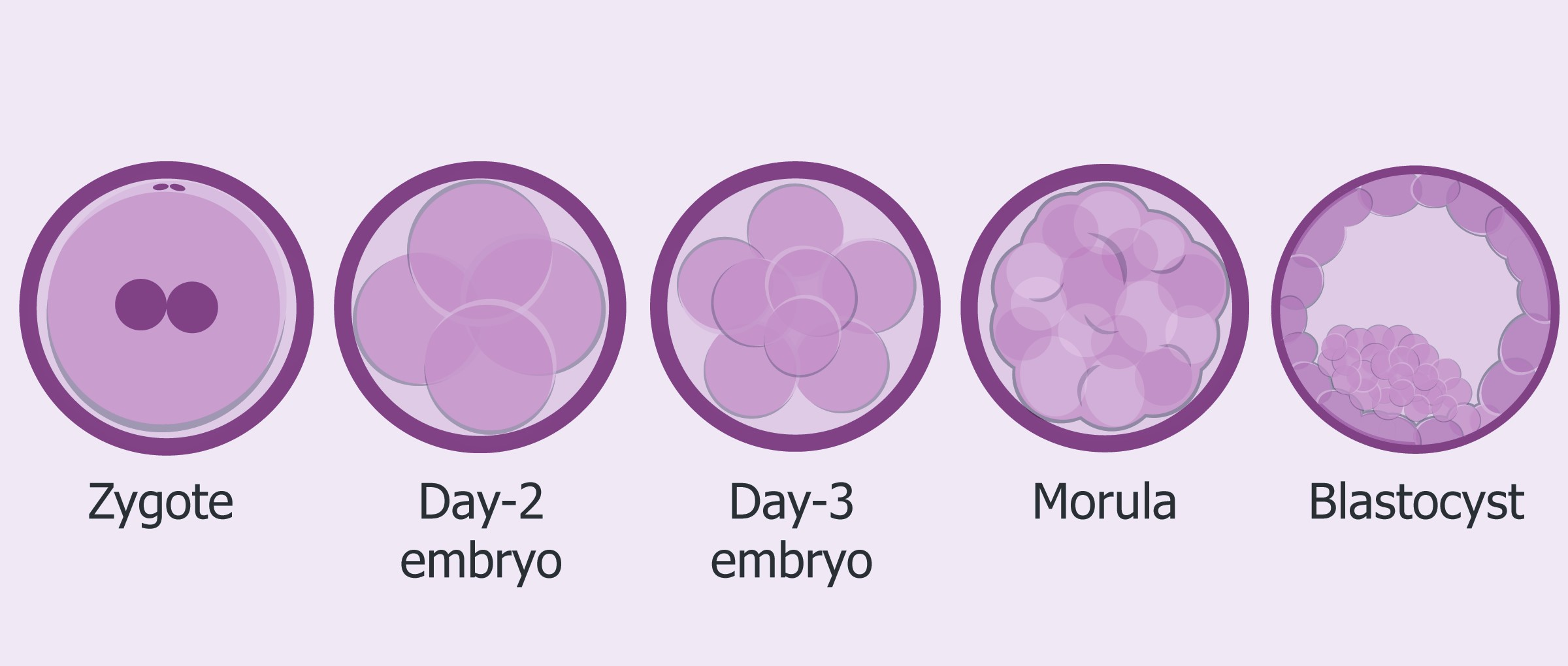
Fig. Embryo development
Any non-viable embryos are discarded. Day 6 is the last day that an embryo can remain in the lab without being transferred or cryopreserved.
Assisted Hatching
What is assisted hatching?
For the first 5 to 7 days of development, the embryo is surrounded and protected by an outer shell called the zona pellucida. In normal circumstances, when the embryo reaches the uterus, this zona partially dissolves and the embryo ‘hatches’ out, allowing it to implant in the uterus.
In some patients it is thought that infertility may be caused by a hardening of the zona, which makes it difficult for the embryo to hatch and implant.
Assisted hatching is a laboratory micromanipulation technique carried out before the embryos are replaced in the uterus that helps the embryo to hatch from the zona.
What does assisted hatching involve?
Assisted hatching is carried out in the laboratory by experienced embryologists. Using a very high powered microscope, a small slot is made in the zona using a very fine needle. Assisted hatching is carried out before the embryo transfer on those embryos that have been chosen for transfer.
Assisted hatching is carried out in the laboratory by experienced embryologists. Using a very high powered microscope, a small slot is made in the zona using a very fine needle. Assisted hatching is carried out before the embryo transfer on those embryos that have been chosen for transfer.
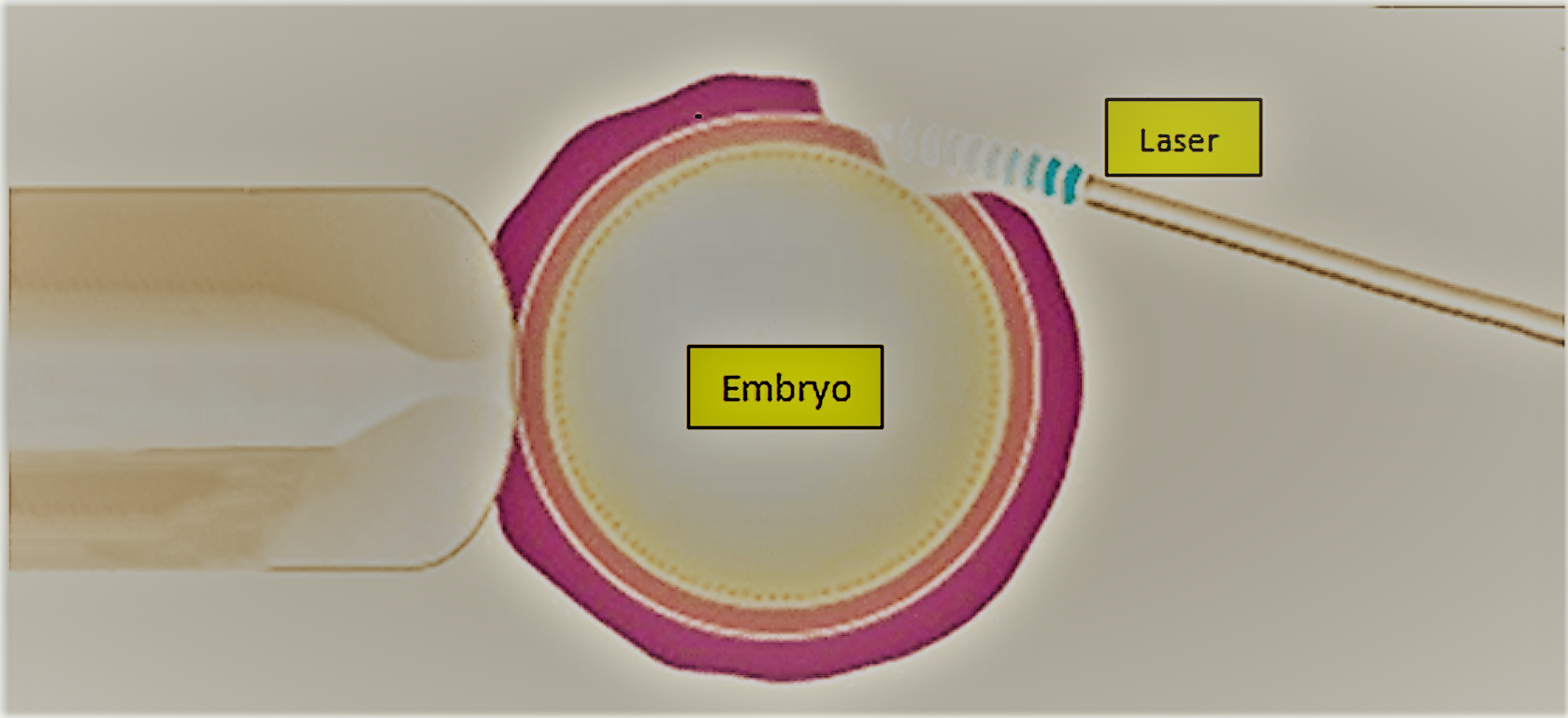
Fig. Assisted hatching
Who is assisted hatching suitable for?
Assisted hatching is generally recommended in the following circumstances:
- The woman has decreaed ovarian reserve
- Couples who have failed to get pregnant following previous IVF cycles
- Couples where a distinct thickening of the zona is noted by the embryologist.
- Frozen embryo replacements.
Preimplantation genetic diagnosis (PGD)
What is PGD?
Preimplantation genetic diagnosis (PGD) is a reproductive technology used with an IVF cycle to increase the potential for a successful pregnancy and delivery. It is a genetic test on cells removed from embryos, to help select the best embryo(s) for pregnancy or to be free of a genetic disease.
Who should consider PGD?
PGD may be considered in all IVF cycles; however, those who might benefit most from this test are couples at increased risk for chromosome abnormalities or specific genetic diseases. This includes women who have had several miscarriages, or who have had a prior pregnancy with a chromosome abnormality. Women over 38 years of age and men with some types of sperm abnormalities may also produce embryos with higher rates of chromosome abnormalities. In addition, if a person carries a rearrangement of the chromosomes, PGD can identify which embryos have a normal amount of chromosomal material. When there is a 25% or 50% chance to have a child affected with a specific genetic disease, PGD can be designed to identify which embryos are affected, unaffected, or a carrier (if applicable) for that disease. Then, only embryos without the disease are transferred to the uterus to attempt pregnancy.
What are the PGD steps during the IVF cycle?
After embryos are created in the laboratory, they are grown for five to six days. On day 3 or in day 5, the biopsy for PGD is done on all appropriately developing embryos.
In the case of PGD on day 3, biopsy involves removing one cell from the embryo; this cell will be genetically tested and will carry the genetic make up of the embryo. Our team will discuss PGD test results with the woman/couple, and a fresh embryo transfer on day 5 will be planned. Only the genetically normal embryos will be transferred.
In the case of PGD on day 5, biopsy involves removing a one few cells from the trophectoderm, or the layer of cells that is ‘hatching out’ of the embryo at this stage of development. The embryos are stored while genetic material inside the removed cells is tested for abnormalities. Our team will discuss PGD test results with the woman/couple, and a frozen embryo transfer (FET) cycle is planned for use of the embryo(s). Only the genetically normal embryos will be transferred.
Is biopsy and PGD safe?
Yes. In embryos where chromosomal microarray testing is performed, one can expect fewer pregnancies with chromosomal disorders since most chromosomal disorders are identified prior to transfer of the embryos to the uterus. Removal of a few of the cells of the early embryo does not alter the ability of that embryo to develop into a complete, normal pregnancy.
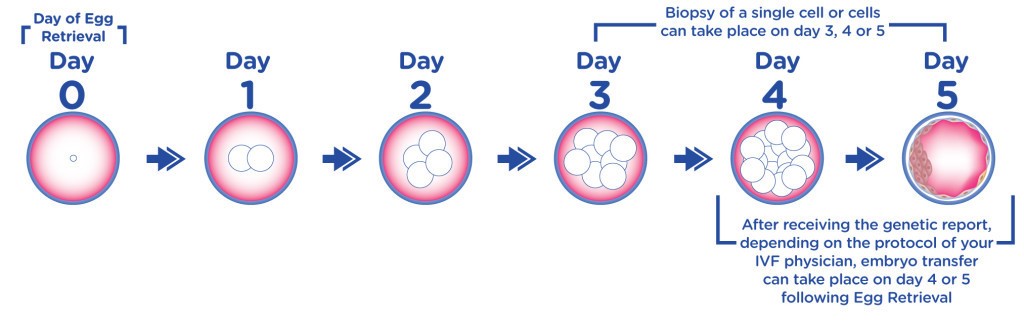
Fig. Preimplantation genetic diagnosis
How are embryos chosen for transfer?
Only the genetically and morphologicaly normal embryos will be transferred. While embryo morphology is helpful in selecting the best embryos for transfer, it is known that many embryos with significant chromosome abnormalities have normal morphology. Therefore, PGD results help to better identify the best embryos to transfer to the uterus. The combination of normal genetic testing with normal physical appearance indicates the highest chance of becoming a healthy pregnancy. All decisions regarding which embryo(s) to transfer to the uterus and how to use the remaining embryos are made together between the couple and their medical team.
Time lapse imaging - Embryoscope
The selection of embryos for transfer is crucial to the success of IVF - both for a healthy delivery and to ensure a singleton pregnancy. Now, in certain cases and for certain patients, embryo selection can be helped by a new technique which visualises the progress of embryo development with a photograph taken every ten minutes.
image src="/wp-content/uploads/2018/03/Time-lapse-imaging-of-the-embryos.png" width="" height="" align="center" border="1" margin_top="" margin_bottom="" link="" link_image="" target="" alt="" caption="Fig. Time lapse imaging of the embryos" greyscale="0" animate=""]This new technique of 'time-lapse imaging' is being quickly adopted by IVF clinics throughout the world. The time lapse technology allows them to inspect the development of their embryos with great frequency but without disturbing them or exposing them to outside air conditions. So here at the LWC we can now culture embryos and monitor their progress in totally safe and stable conditions, with an image of their composition at every tiny stage of development.
Studies so far suggest that embryos selected with the help of time-lapse imaging have a high chance of forming a healthy pregnancy, so the technology will be especially welcome for patients with a poor reproductive record - that is, women who have already been unsuccessful in IVF and/or those of an older reproductive age.
The information provided by time lapse technology and the criteria by which embryos are selected for transfer relates mainly to the rate at which the embryos progress from embryonic stage to the next - so the time between these stages is crucial. It is the rapid sequence of images available to the embryologist which allows analysis of these changes, and thus the assessment of which embryo has the best chance of implantation in the uterus.
Embryo Grading. After insemination by IVF or ICSI, fertilised eggs are the routinely cultured in the laboratory for 3 - 5 days before being transferred back into the woman’s uterus. Before embryo transfer, the embryos are graded by specially trained embryologist in order to select the embryos with the best chance of implanting in the uterus and forming a healthy baby.
Embryo transfer procedure. If the embryos have developed normally after incubation, one of our IVF specialist transfers a predetermined number of embryos through the cervix into the uterus via a small catheter (hollow tube). The patient and her physician determine the number of embryos for transfer based on individual circumstances such as age and medical history. No anesthesia is required for this procedure, although Valium or Piroxicam is given for uterine relaxation. If any excess embryos exist after the initial transfer, the patient may request evaluation for possible freezing and use for a subsequent treatment cycle.
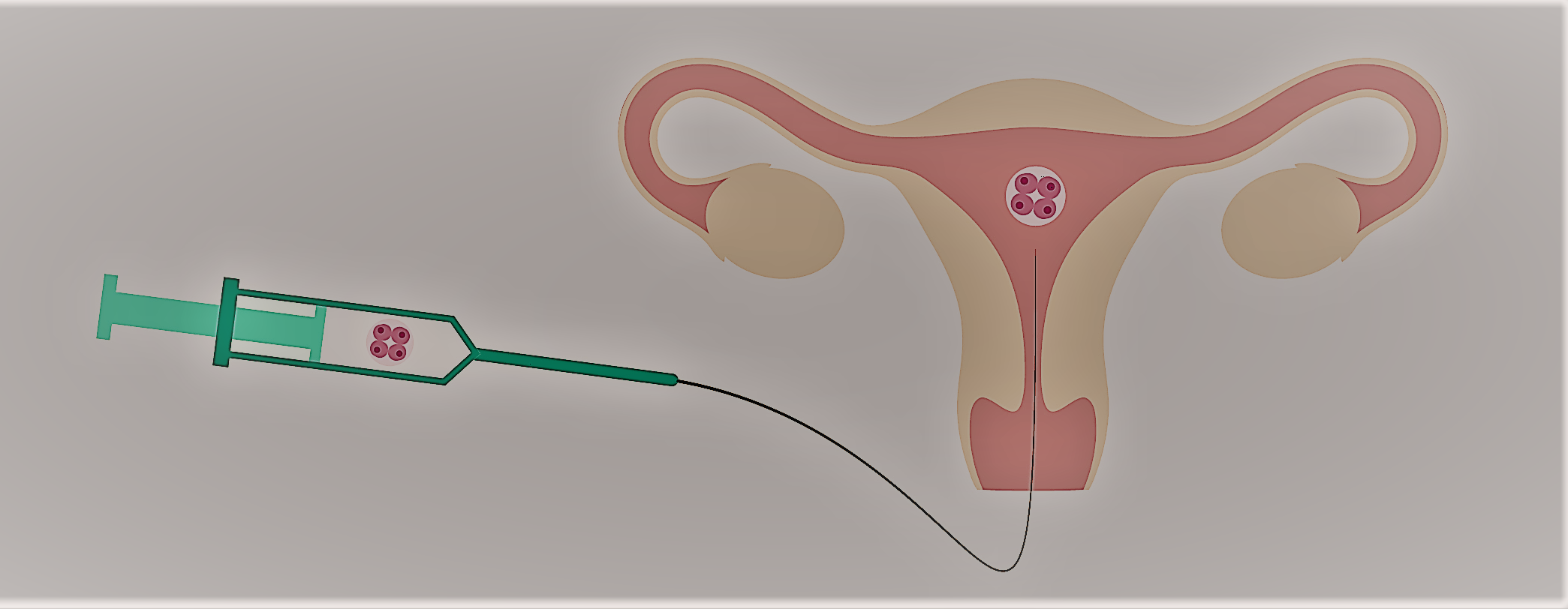
Fig. Embryo Tranfer
Progesterone supplement. In a normal fertility cycle, following ovulation, the body will produce the hormone progesterone, which serves to thicken the uterine lining and help an egg to implant and grow, thus maintaining an early pregnancy. In an IVF cycle, progesterone is taken most commonly by intra-vaginal insertion of micronized progesterone and by intra-muscular injection. Progesterone is continued for two weeks, when the patient receives a pregnancy test and continued when pregnant until the 8th – 12th wk of pregnancy.
Estrogen supplement. When women take the IVF stimulation medications, her estrogen levels will be very high at the end of the stimulation phase. They will tend to fall after the egg retrieval. In order to avoid a precipitous fall and risk early uterine bleeding, we usually prescribe estrogen to keep a nice even level during the luteal phase. This estrogen is stopped at the first pregnancy test, regardless of the result.
